HEALTH AGENCY ASKING DEA TO RESCHEDULE CANNABIS
THE PROMINENT FEDERAL HEALTH AGENCY HAS SUGGESTED A HISTORIC RECOMMENDATION TO THE DEA, PROPOSING THAT MARIJUANA SHOULD BE RECLASSIFIED TO SCHEDULE 3. READ MORE TO FIND OUT WHAT THIS MEANS!

The U.S. Department of Health and Human Services (HHS) has officially proposed a significant change, suggesting that marijuana's classification be shifted from Schedule I to Schedule III according to federal law. This groundbreaking development signifies that the leading health agency no longer views cannabis as a substance with high potential for abuse and no medicinal merit.Following a scientific evaluation of cannabis prompted by a directive from President Joe Biden the previous year, the HHS is now communicating to the Drug Enforcement Administration (DEA) its belief that marijuana should be re-positioned to Schedule III within the Controlled Substances Act, as initially reported by Bloomberg. While the recommendation doesn't have binding force, the DEA ultimately holds the decision-making authority. Nevertheless, the scientific assessment could strongly influence the DEA's decision to enact the change.Under Schedule III, cannabis would still be federally prohibited. However, this rescheduling would bring about significant consequences for researchers who have long criticized the restrictive barriers to access imposed by the current Schedule I categorization, thereby enhancing opportunities for studies.Moreover, reclassifying cannabis to Schedule III would open up potential tax advantages within the marijuana industry that are presently not accessible.HHS Secretary Xavier Becerra indicated to Marijuana Moment in June that his agency aimed to conclude the evaluation by the year's end.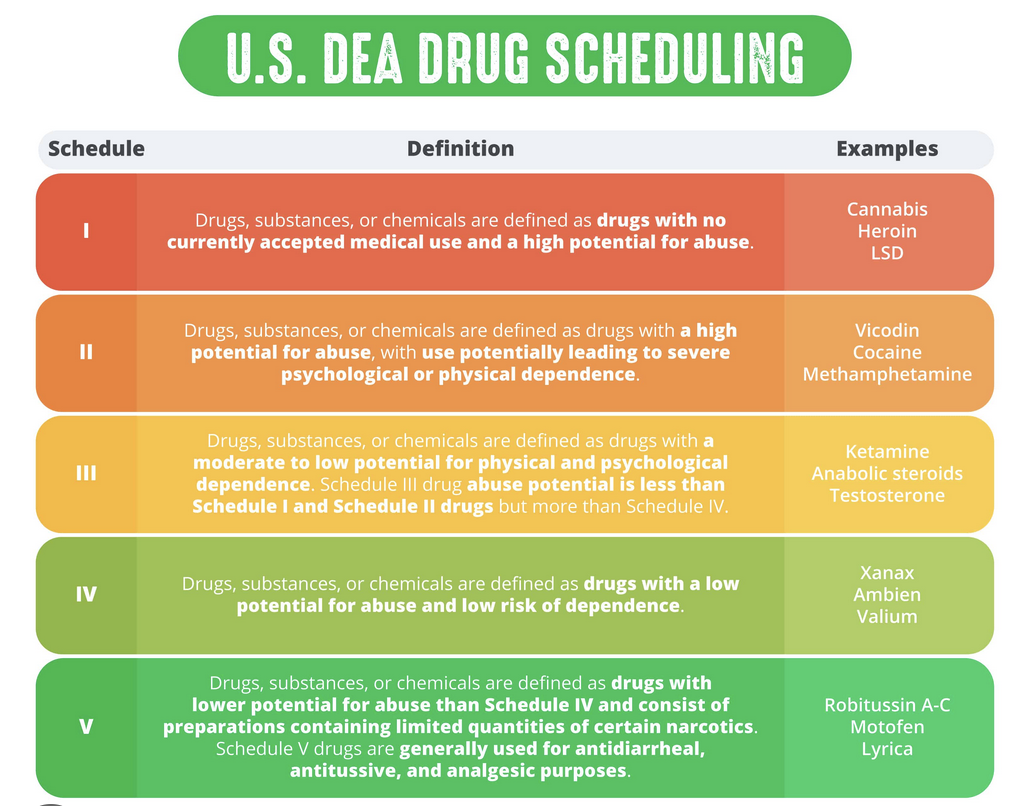
DRUG SCHEDULES EXPLAINED
Enacted in 1970, the Controlled Substances Act categorizes narcotic substances into five distinct groups known as "schedules." The primary responsibility for classifying and assessing these substances lies with the Drug Enforcement Administration (DEA). The assignment of drugs to specific schedules is determined by factors such as their potential for abuse, legal status, and recognized medical uses. Schedule I encompasses drugs deemed to carry the greatest risks, while Schedule V includes narcotics with a relatively lower likelihood of causing addiction.The list's designations have sparked controversy, particularly in relation to marijuana's classification as a Schedule I drug. Critics of this categorization have emphasized marijuana's medicinal benefits and questioned its placement in a higher schedule compared to substances like heroin or cocaine, which have a greater potential for abuse. Various efforts have emerged over the years to reclassify marijuana away from Schedule I, but thus far, none have succeeded. Nevertheless, the legal status of marijuana has shifted significantly, as it is now permitted for medicinal or recreational use in the majority of states.
SCHEDULE ORGANIZATION
HERE ARE SOME OF THE DRUGS BELONGING IN EACH SCHEDULE:
SCHEDULE I: MARIJUANA, ECSTASY, HEROIN, LSD, AND PEYOTE
SCHEDULE II: METHAMPHETAMINE, COCAINE, FENTANYL, VICODIN, OXYCODONE, AND ADDERALL
SCHEDULE III: ANABOLIC STEROIDS, TESTOSTERONE, AND KETAMINE
SCHEDULE IV: XANAX, AMBIEN, ATIVAN, AND VALIUM
SCHEDULE V: COUGH SUPPRESSANTS
SOURCE: HTTPS://WWW.FINDLAW.COM/LEGALBLOGS/LAW-AND-LIFE/DRUG-SCHEDULES-EXPLAINED/


